
Vous êtes :
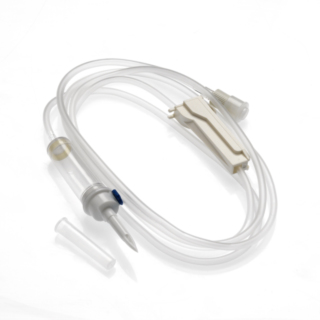
The DEHP-free, BPA-free INFINEED lt;Gravity lt; 1-way infusion set was designed to minimize the device's environmental impact. Two methods have been used: first, by reducing the plastic mass used by around 10%, without making any changes to functionality; second, by exclusively using raw materials that are free from endocrine disruptors (elimination of components comprising DEHP or BPA.)
- Two-channel perforator
- Air intake: Delivered closed for immediate use on a 0.2µm filter bag.
- Chamber: 5.5 mL / 5 cm long - Flexible and transparent, Calibrated at 20 drops = 1ml ± 0.1ml, with 15µm particle filter.
- Flow regulator: ABS linear flow control for good roller clamp rigidity, precision and reliability.
- Tubing: PVC (DINCH) designed for comfort, optimal flow adjustment consistency and to prevent kinks.
- End fitting: Fixed Luer Lock
- End stopper: Equipped with a very pressure-resistant purge filter with antibacterial membrane of 0.8µm.
Length: 160 cm
Residual volume: 15 ml
Made in China
Contact with 0.5% or 2% Chlorhexidine in 70° Isopropyl alcohol, without
evaporation time, and while the luer lock is connected, therefore under
physical stress, presents a significant risk of cracking of the luer
lock, and should be avoided as much as possible.
In
accordance with the recommendations of the SF2H (2019), disinfecting the
connectors (when necessary) with 70° ethyl alcohol is always
preferable, as it is just as effective in disinfecting inert materials
and there is much less risk of altering plastic materials.
Indications
This device enables parenteral administration of injectable preparations by gravity.
For peripheral lines, the infusion line can remain in place for a maximum of 96 hours (i.e. the maximum time the peripheral catheter can be in place).
For central lines, the infusion line must be changed at least every 72 hours (or according to the institution's validated protocol).
Dispose according to legislation in member state.
Matériaux utilisés
PVC (DEHP< 01.% (m/>)): Chamber, air intake, tubing - DINCH: plasticiser - Polyethylene: Purge filter, protective cap for perforator, mobile Luer Lock - Acrylonitrile Butadiene Styrene: Perforator, linear flow regulator - Polyamide: Particle filter
Étiquetage
on each unit are written the reference, the lot/serial number, the sterilization method and the expiry date if applicable
dispositif médical
stérile
conformité
Conditions de transport
Protected from light, humidity and heat
| Conditionnement | Quantités de pièces | Dimensions en cm | Poids en kg | Ean 13 du conditionnement |
| UNIT | 1 | 3661809029370 | ||
| BOX | 25 | 3661809129377 | ||
| CASE | 250 | 3661809229374 |